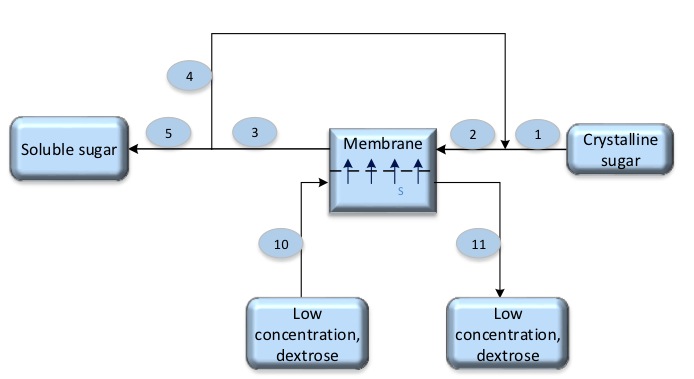
Can forward osmosis systems be used for process optimization in white Biotech?
- The concentration of stream No-2 is 70% Ds.
- The concentration of stream No-3 is 60% Ds.
- The concentration of stream No-10 is 19% Ds.
- The concentration of stream No-11 is 23% Ds.
According to Forward-osmosis the water will pass through membrane from low concentrate (dextrose 19%) to high concentrate (sucrose which is about 70% to 60%)
My questions:
Refer to osmotic pressure of Sucrose and Dextrose in those concentration, it makes sense to build such of system?
- Do you know about the names of producer which can supply such systems?
- Do you know about the names of producer which can supply such systems for sugar syrup?
- You can estimate the pressure of streams No 10,11?
- What is the flow rate of the water which pass through the membrane: liter/m^2/h?
ForwardOsmosisTech is always happy to answer forward osmosis related questions. Especially when the questions are related to industrial forward osmosis applications:
Question 1: Refer to osmotic pressure of sucrose and dextrose in those concentration, it makes sense to build such of system?
Osmotic pressures ∏ of dilute solutions can be approximated using the Morse equation as described in our article “The principles of forward osmosis“:
∏ = iRTM
- i is The Van’t Hoff factor, which reflects the dissociation multiple of the solute species in question. For a dilute solution of sodium chloride, the Van’t Hoff factor is equal to 2 because 1 mole of NaCl dissociates into 2 moles of solutes in aqueous solution.
- R is the gas constant in L*atm*K-1*M-1
- T is the temperature of the solution in Kelvin [K]
- M is the molarity of the solution in Molar [M]
As indicated earlier, the Galam-Group are working with concentrated dextrose and sucrose solutions (concentrations varying from 19% Ds (dry substance) to 70% Ds (dry substance)) and the osmotic pressures of these solutions can only be roughly estimated by the Morse equation. For exact calculations of osmotic pressures of concentrated solutions we refer to specialized software from vendors such as OLI systems. Under the assumption that the dry substance present is either dextrose or sucrose with no contaminations, the Morse equation yields the following osmotic pressures:
| Solution | Concentration (g/l) | Molarity (M) | Osmotic pressure (bar) |
| 70% Ds. sucrose | 700 | 2,0 | 51 |
| 60% Ds. sucrose | 600 | 1,8 | 43 |
| 19% Ds. dextrose | 190 | 1,0 | 26 |
| 23% Ds. dextrose | 230 | 1,3 | 32 |
Input: M (sucrose) = 342,3 g/m0l ; M (dextrose) = 180,2 g/mol ; Van’t hoff factor = 1 ; Gas constant (R) = 0,08314 l*bar*K ; Temperature = 298K (25ºC)
Based on the osmotic pressures summarized in the table above, the osmotic pressure differences (i.e. driving forces) for the process proposed by the Galam-Group will range from 11 (43-32) to 25 (51-26) bar inside the forward osmosis membrane module.
From a forward osmosis perspective this is a relatively low osmotic pressure difference, which will make it difficult to achieve high water flow rates across the membranes in the system.
Whether or not the system makes sense for the Galam-Group to build therefore depends on their requirements with regards to system footprint and performance.
Question 2: Do you know about the names of producer which can supply such systems?
Based on our understanding of the current commercialization status of forward osmosis membrane system providers, the five main players are Oasys, Trevi Systems, Modern Water, Hydration Technology Innovations (HTI), and Porifera.
Question 3: Do you know about the names of producer which can supply such systems for sugar syrup?
For the sugar syrup forward osmosis application we believe the most suitable system provider is either Trevi Systems or Hydration Technology Innovations.
Question 4: You can estimate the pressure of streams No 10,11?
Based on our rough estimations, the osmotic pressures of 19% Ds. and 23% Ds. dextrose are 26 and 32 bar respectively.
Question 5: What is the flow rate of the water which pass through the membrane: liter/m^2/h?
This is a difficult question to answer precisely since the water flow depends on both physical parameters of the forward osmosis membranes in use as well as the design and operating conditions of the forward osmosis system. For solutes such as dextrose and sucrose, which have diffusion coefficients that are considerably smaller (0,673*10-9 m2/s and 0,46*10-10 m2/s respectively) than sodium chloride (1,61*10-9 m2/s) – a commonly used draw solute in forward osmosis applications – concentration polarization effects will have a strong negative influence on the achievable water flow rates.
Based on our extensive forward osmosis experience, our best guestimate is that current forward osmosis membranes on the market will be able to generate water flow rates in the region 1-5 l/m2h.
As mentioned under question 1, whether or not this flow rate makes sense for the Galam-Group, depends on their system footprint and time requirements. Installing more membrane area and allowing the sucrose dilution process to extend over longer time both compensate for low water flow rates.