- Nano-selective forward osmosis technology by SideStroem featured in Everything About Water - February 10, 2024
- SideStroem and Singapore Institute of Technology featured in AsianScientist - April 29, 2023
- SideStroem joins world class accelerator program - November 12, 2022
Sourcing of commercially available Pressure Retarded Osmosis (PRO) membranes for research purposes?
Recently, we received an inquiry from Lamar regarding the best commercial source of PRO membranes:
Greetings,
I am trying to find the best commercially available PRO membranes for my research. The membranes need to hold up to approx 400 psi pressure and have as high of a flux and rejection rate as possible. Any assistance is greatly appreciated.
Kind regards,
Lamar Small
Unfortunately, Lamar, PRO membranes are not commercially available today. Two alternative means of securing your research foundation come to mind:
- Buy regular FO membranes instead. Although the PRO performance of regular FO membranes may be limited, you should still be able to get meaningful data from the experiments.
- Contact various university groups working on PRO membranes and request for samples. You should be prepared to share your research results with the group(s) you get samples from. To our knowledge there are several research groups working on PRO membranes in Singapore, Japan, and South Korea.
In any case, it would be interesting for us to know what kind of PRO membrane sample size and form factor you will be needing for your research.
-0-

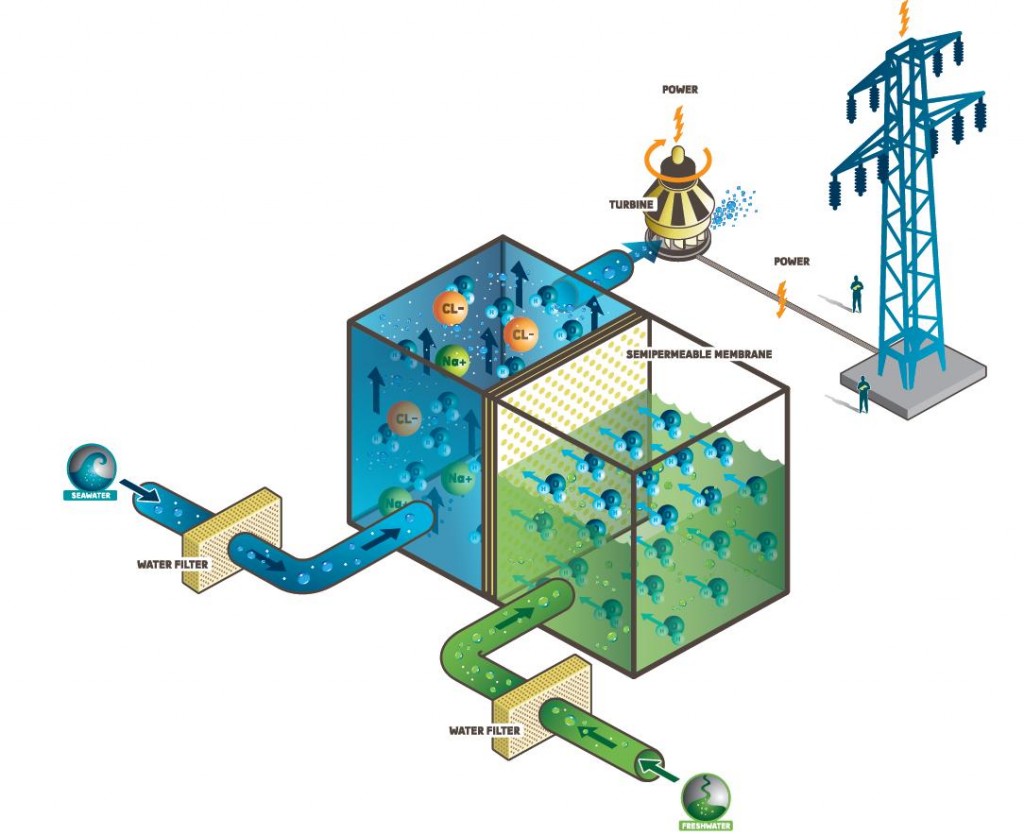
Greetings,
I recently conducted FO experiments using flat sheet membranes. I noticed that the water flux decreased over time. Intially it was quite high but after 15 min, it started to decrease over time. I ran the experiment for 45 min after removing all the air bubbles from the system. I checked the draw solution concentration. No obvious dilution took place throughout the experiment.
Why this happened? Is it typical to observe this trend in FO?
Thank you in advanced.
Sincerely,
Lim
Hi Lim
Thanks for reaching out on this. In order to answer your question, I need a bit more information:
– What was the composition of your feed & draw solutions in the experiments?
– What kind of flow cell are you using?
– What was the linear flow velocity across the membrane in the experiments?
– What was the initial reverse salt flux, and did the reverse salt flux also change over time?
Cheers
Mark